What are you looking for?
Antagonist
Antagonist
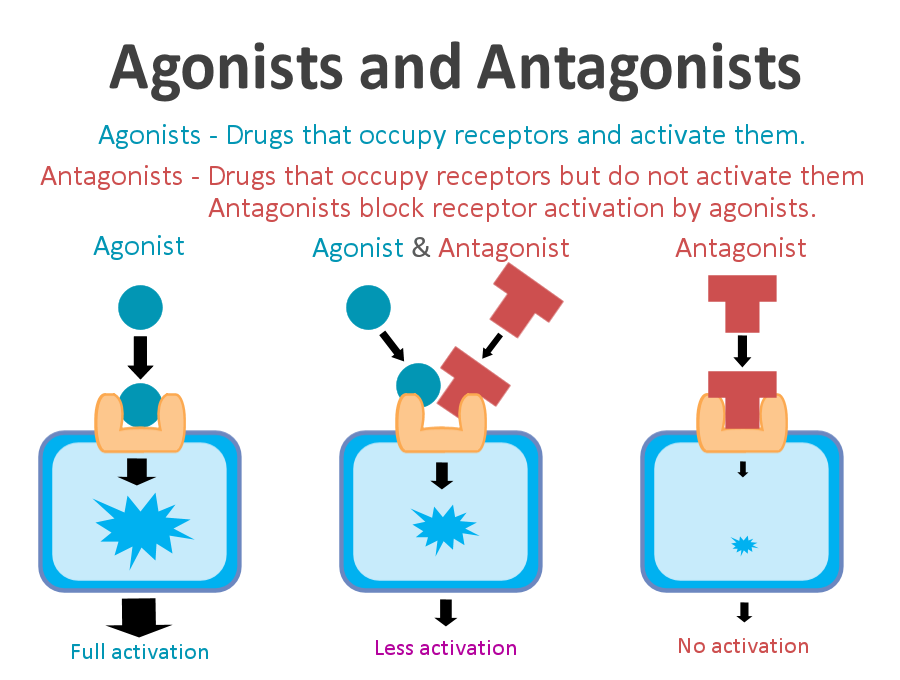
A receptor antagonist is a chemical that binds to a receptor of a cell, but does not trigger a response by that cell. Antagonists have affinity towards binding to the receptors they target, but no efficacy to activate the receptor.[1][2] Receptor antagonists have no activity in the absence of a receptor agonist; however, the binding of a receptor antagonist will disrupt the interaction and inhibit the function of an agonist or inverse agonist at receptors.
Antagonists mediate their effects by binding to the receptors they target at various possible locations. Some antagonists target the same binding site as the receptor agonist while some bind to allosteric sites on receptors; antagonists may also interact at unique binding sites not normally involved in the regulation of a receptor’s activity. The binding of a receptor antagonist to the receptor it targets may be permanent or temporary depending on the nature of the bond formed.
Antagonists can be subdivided into competitive or non-competitive and reversible or irreversible, depending on the location and nature of their binding to a receptor.
Types of antagonists
Competitive antagonists
Competitive antagonists reversibly bind to receptors at the same binding site as the endogenous ligand or agonist without activating the receptor. Competitive antagonists compete with agonists for the same binding site on the receptor. While bound to the receptor, a competitive antagonist will block agonist binding.
The degree of activity of the target receptor in the presence of an agonist and a competitive antagonist is a function of the relative affinity each has for the active binding site and their respective concentrations. Controlling for concentration, the drug with higher affinity will occupy a greater number of receptors than the drug with lower affinity. The ability of a competitive antagonist to block the effects of an agonist can be reduced or eliminated by sufficiently increasing the concentration of the receptor agonist.[3] Therefore, competitive antagonists do not reduce the maximum response of a receptor agonist, but do increase the agonist dosage required to achieve it.[4]
Naloxone is an example of a competitive receptor antagonist which targets opioid receptors. Naloxone achieves its effects by having a much higher affinity for opioid receptors than opioid agonists, thus out-competing the agonist for receptor occupancy.[5][6]
Irreversible competitive antagonists
Irreversible competitive antagonists target the active site of a receptor, initially competing with receptor agonists to bind to the receptor. However, once bound, an irreversible competitive antagonist permanently deactivates the receptor.[7] Deactivated receptors cannot be affected by any amount of a receptor agonist, therefore lowering the greatest possible effects agonists can have on a receptor set until the body synthesizes new receptors. The duration of an irreversible antagonist is dependent on the rate of synthesis of the target receptor set, which varies by receptor type. Irreversible competitive antagonists are sometimes classified as non-competitive antagonists for their ability to reduce the maximum achievable effects of agonists.[8]
α-Neurotoxins, found in certain families of venomous snakes, are an example of irreversible competitive antagonism. α-Neurotoxins bind competitively and irreversibly to nicotinic acetylcholine receptors (nAChRs) found in neuromuscular synapses.[9] Irreversible competitive antagonists are also used in medicine, such as naloxonazine (not to be confused with naloxone). Naloxonazine binds irreversibly to μ-opioid receptors through covalent bonding, making it impossible for the molecule to unbind and blocking the receptor permanently.
Non-competitive antagonists
A non-competitive antagonist binds to the receptor it targets at an allosteric site, or a site other than the active site an agonist binds to. This type of binding is non-competitive because the receptor antagonist does not have to compete with agonists for binding. A non-competitive antagonist produces its effects by inducing a conformation change in the receptor which changes the shape of the active binding site, preventing agonists from binding. Non-competitive antagonism cannot be negated regardless of how much agonist is present, therefore reducing the maximum possible effects of a receptor agonist for the duration the antagonist remains bound to a receptor. Non-competitive antagonists are generally reversible, but may also be irreversible.
Ketamine is an example of a non-competitive antagonist of NMDA receptors.[10]
Partial agonists
Partial agonists may act as a competitive antagonist in the presence of a full agonist. Partial agonists have lower efficacy for the receptors they target than full agonists, therefore producing weaker effects. In the presence of a partial agonist, the full agonist must compete for receptor occupancy. This results in some receptors being occupied by the partial agonist and some by the full agonist, generating a weaker overall response than would be produced by the full agonist alone. The effects of a partial agonist can be overcome by increasing the dosage of the full agonist.
Buprenorphine is a partial agonist to the μ-opioid receptor commonly used to treat opioid addiction in higher doses. Alone, it is a weak agonist to the μ-opioid receptor with a high affinity and low efficacy; however, in the presence of other opioid drugs, buprenorphine has antagonistic effects. A sufficient dose of buprenorphine may diminish or negate the additional effects of even heavy doses of codeine, morphine, or heroin by blocking them from binding to μ-opioid receptors.[11] It is very difficult to achieve medicinal opioid analgesia in patients on buprenorphine, a drug with higher affinity is required to out-compete it to bind to μ-opioid receptors, such as fentanyl or its analogues.[12]